When we look at objects giving off light (or reflecting light) we find three types of spectra. They are produced in different ways, and can actually tell us about the physical properties of the materials producing the spectra. In many ways it was the development of studying and understanding the spectra of astronomical objects that led to the development of astrophysics as opposed to the more traditional astronomy. This happened from the mid 1800s.
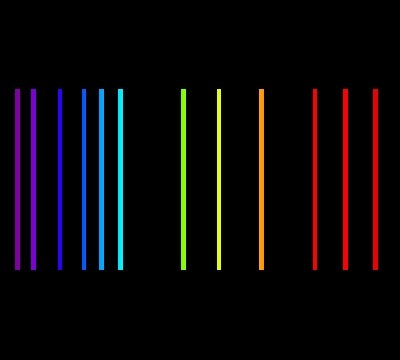
The three types of spectra are called “a continuous spectrum” (or continuum emission), “an emission line spectrum” and “an absorption line spectrum”. They look like the following
UPDATE
You can read about these three types of spectra in more detail in my new book – see http://www.springer.com/astronomy/popular+astronomy/book/978-3-319-09927-9
A continuous spectrum
When Newton did his famous experiment with a prism and sunlight, he noted that the Sun produced a “rainbow” of colours. This is a continuous spectrum. (However, as I will discuss in a future blog, if he had been able to produce a more detailed spectrum he would have noticed some subtleties on this continuous spectrum). So, light from the Sun, and any star, produces a continuous spectrum.
We also get a continuum spectrum from a hot solid, so for example the light produced by incandescent light bulbs is a continuum spectrum. These kinds of bulbs give off light by a very thin coil of metal, the filament, (usually tungsten) getting extremely hot from having an electric current passed through it. When the filament gets to thousands of degrees, it gives off light.
An emission line spectrum
If, instead of looking at the spectrum of the Sun we were to look at the spectrum of an object like Messier 42 (the Orion nebula), we would notice a very different kind of spectrum. Rather than being a continuous spectrum, we would see a series of bright lines with a dark background. We would also see an emission line spectrum if we were to look at the spectrum from one of the fluorescent light sources which are now replacing the incandescent lights in houses.
UPDATE
You can read about these three types of spectra in more detail in my new book – see http://www.springer.com/astronomy/popular+astronomy/book/978-3-319-09927-9
An absorption line spectrum
An absorption line spectrum is in some ways the converse of an emission line spectrum. Rather than seeing a series of bright lines on a dark background, one sees dark lines on a continuous spectrum.
Putting it all together
The diagram below shows how the three types of spectra can be produced. If we observe a continuum source (such as a star of an incandescent light) with nothing between us and that source then we will see a continuous spectrum.
If, instead, we look at a gas cloud then we will see an emission line spectrum. This is why the Orion nebula has an emission line spectrum, because we are seeing the emission from the gas cloud from which the stars have formed and still are forming. The lines are in particular places on the spectrum which depends on the composition, pressure and temperature of the gas, as well as whether it is moving towards us or away from us.
If we look at the same gas cloud but with a continuum source behind the cloud then we will see an absorption line spectrum. The dark lines are in exactly the same places (at the same wavelengths) as for the emission line spectrum, but are dark rather than bright.
This was observed by physicists as early as the 1850s. In fact the diagram above is known as Kirchhoff’s radiation laws, after Gustav Kirchhoff (1824-1887), a German physicist of the time. He and Robert Bunsen (he of the eponymous burner) did important spectroscopy work in the 1850s and 1860s. But it was actually not until the 1920s that physicists properly understood the physics of these three different kinds of spectra. I will explain this physics in a series of future blogs.
UPDATE
You can read about these three types of spectra in more detail in my new book – see http://www.springer.com/astronomy/popular+astronomy/book/978-3-319-09927-9

My book, “The Cosmic Microwave Background” includes Fraunhofer’s sketch of the first ever absorption spectrum seen of the Sun, and an explanation of why stars have different colours.




[…] and M1 were all examples of objects which exhibited an emission line spectrum. And in my blog on the three kinds of spectra, of course emission line spectra are one of the three types. So, how do emission line spectra come […]
[…] to be due to absorption lines being produced by different elements. I discussed absorption spectra in this blog. We now know that the gases which produce the absorption lines are in the atmosphere of the Sun. […]
wow
[…] will have certain wavelengths removed, this is the principle behind the absorption spectrum that I explained here. The scientists have found that wavelengths corresponding to water vapour have been removed from […]
Does all light, e.g., the light from the sun or a lamp, consist of or include all three spectra, or does light only consist of or include one spectrum depending upon what generated the light (i.e., cool gas vs. hot lamp or sun)?
Light from the Sun looks like a continuum spectrum, but has dark lines (absorption lines) in it, which Fraunhofer discovered in 1814-15.
An incandescent lamp has a continuum spectrum, a fluorescent lamp an emission line spectrum.
A heated cannonball glowing red would have a continuum spectrum. A neon light an emission line spectrum.
I hope this answers your question.
Thank you.
Qn.what is the significance of covergence limit in relation to chemical bonding
I’ve no idea, I’m not a chemist.
alright,thanks any way i hope i’ll get 1 soon
I didnt understand exactly the formation of those spectra
Which parts would you like me to clarify?
[…] something I have blogged about. For example, here in a blog entitled Emission Line Spectra, and here in my basic explanation of the three kinds of spectra we see in nature. It is the kind of emission given off by e.g. the […]
I want to know more about continuous spectrum .
Such as?
You mention only examples for continuous spectrum but i am not understand what is the meaning of continuous spectrum . Can you tell me more about it.
A continuous spectrum is emitted by solid, liquid or opaque gas. It occurs when the radiation is in thermal equilibrium with the material.
Thanks alot
You’re welcome
My topic is about identification of elements by spectrum.
Can u help me with this.
Each chemical element and each element’s ions have unique spectra, allowing us from its emission or absorption spectrum to identify the element and its ionic state.
[…] bulb or an electric stove burner give off every color of light and emit a line-free, continuous spectrum that looks like a rainbow. A star is likewise hot and dense and produces a continuous […]
how are the three types of spectra defined?
Read the blog, they are defined by their different appearances.
how are the distances between stars measured?
By a variety of methods. The most direct is stellar parallax.
just wondering.
Which type of spectrum is produced by an incandescent bulb?
A continuum spectrum.
If you were looking at the spectra of light coming from the Sun (or any stars), which of the three types of spectrum would be observed?
An absorption spectrum. The absorption lines are produced by the gases in the atmosphere of the Sun (or stars).
what did you think, Rhodria?
Think of what??
when do you exactly get an absorption spectrum? And incandescent light gives off different wavelengths of light, so why don’t we see an emission spectrum there?
Read the blogpost again. It’s explained in the blogpost